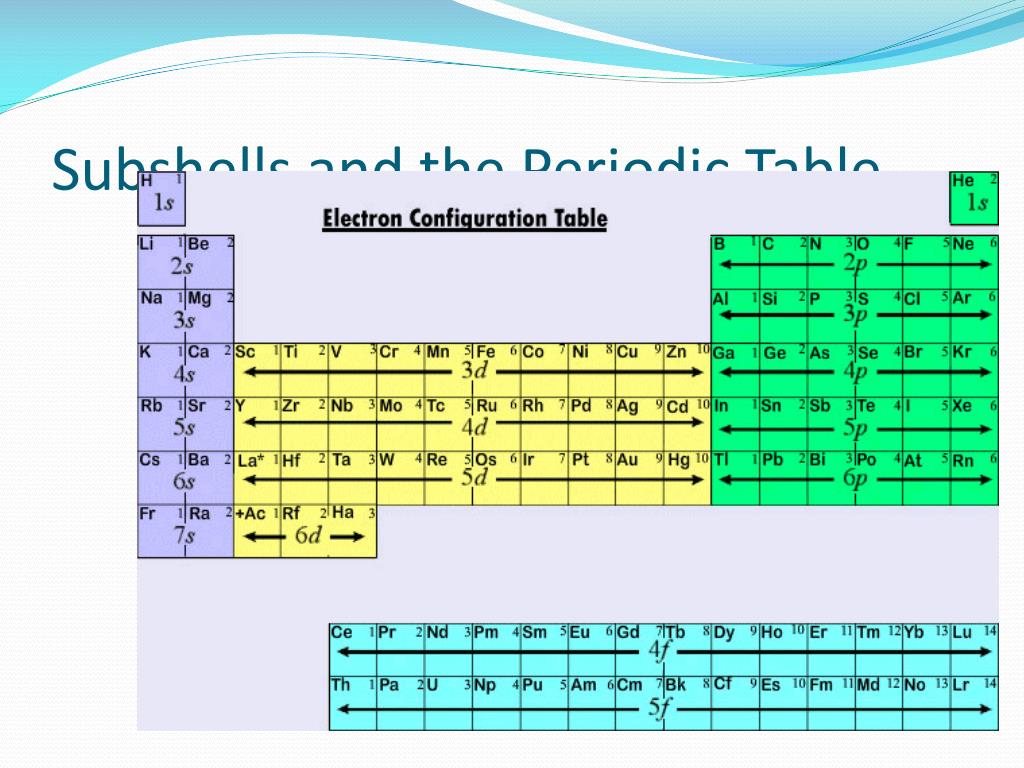
Subshell Blocks Periodic Table . ⚛ group 1 elements occur. the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The shape of the periodic table mimics the filling of the subshells with electrons. the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the answer is rather simple, if you understand electron configurations: the periodic table, electron shells, and orbitals.
from www.slideserve.com
⚛ group 1 elements occur. one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. The shape of the periodic table mimics the filling of the subshells with electrons. the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. the periodic table, electron shells, and orbitals. the answer is rather simple, if you understand electron configurations: based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section.
PPT Chapter 8 Periodic Properties PowerPoint Presentation, free
Subshell Blocks Periodic Table the answer is rather simple, if you understand electron configurations: the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. The shape of the periodic table mimics the filling of the subshells with electrons. the answer is rather simple, if you understand electron configurations: one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. ⚛ group 1 elements occur. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the periodic table, electron shells, and orbitals. the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons.
From slideplayer.com
Electron Configuration ppt download Subshell Blocks Periodic Table ⚛ group 1 elements occur. the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the periodic table, electron shells, and orbitals. The. Subshell Blocks Periodic Table.
From utedzz.blogspot.com
Periodic Table With S P D F Blocks Pdf Periodic Table Timeline Subshell Blocks Periodic Table the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. the answer is rather simple, if you understand electron configurations: The shape of the periodic table mimics the filling of the subshells with electrons. the labels s, p, d and f blocks of the periodic. Subshell Blocks Periodic Table.
From dxooqyxsj.blob.core.windows.net
Block In Periodic Table Electron Configuration at Adam Richards blog Subshell Blocks Periodic Table the answer is rather simple, if you understand electron configurations: the periodic table, electron shells, and orbitals. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The shape of the periodic table mimics the filling of the subshells with electrons. ⚛ group 1 elements. Subshell Blocks Periodic Table.
From sciencenotes.org
Periodic Table Blocks of Elements Subshell Blocks Periodic Table based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the answer is rather simple, if you understand electron configurations: The shape of the periodic table mimics the filling of the subshells with electrons. ⚛ group 1 elements occur. the periodic table is separated into. Subshell Blocks Periodic Table.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Subshell Blocks Periodic Table the periodic table, electron shells, and orbitals. The shape of the periodic table mimics the filling of the subshells with electrons. the answer is rather simple, if you understand electron configurations: the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. based on electron. Subshell Blocks Periodic Table.
From www.slideserve.com
PPT Chapter 8 Periodic Properties PowerPoint Presentation, free Subshell Blocks Periodic Table one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. the answer is rather simple, if you understand electron configurations: the periodic table, electron shells, and orbitals. based on electron configurations, the periodic table can be divided into blocks denoting. Subshell Blocks Periodic Table.
From peersay.weebly.com
Different blocks in periodic table peersay Subshell Blocks Periodic Table the answer is rather simple, if you understand electron configurations: the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. the periodic table, electron shells, and orbitals. The shape of the periodic table mimics the filling of the subshells with electrons. the labels s,. Subshell Blocks Periodic Table.
From www.chemistrylearner.com
sBlock Elements Definition and Characteristics Subshell Blocks Periodic Table the periodic table, electron shells, and orbitals. the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. one way to remember this. Subshell Blocks Periodic Table.
From www.pathwaystochemistry.com
Electron Configurations and Orbital Box Diagrams Pathways to Chemistry Subshell Blocks Periodic Table based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the answer is rather simple, if you understand electron configurations: the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The shape of. Subshell Blocks Periodic Table.
From utedzz.blogspot.com
Modern Periodic Table S P D F Blocks Periodic Table Timeline Subshell Blocks Periodic Table the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. ⚛ group 1 elements occur. the answer is rather simple, if you understand electron configurations: the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in. Subshell Blocks Periodic Table.
From hollyimg07.blogspot.com
Electron Subshell Flow Diagram Periodic Table Blocks Of Elements Subshell Blocks Periodic Table the periodic table, electron shells, and orbitals. The shape of the periodic table mimics the filling of the subshells with electrons. the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. ⚛ group 1 elements occur. based on electron configurations, the periodic table can be. Subshell Blocks Periodic Table.
From www.pinterest.com
s, p, d and fBlock Elements Chemistry notes, Chemistry basics Subshell Blocks Periodic Table ⚛ group 1 elements occur. the answer is rather simple, if you understand electron configurations: the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled. Subshell Blocks Periodic Table.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Subshell Blocks Periodic Table the answer is rather simple, if you understand electron configurations: The shape of the periodic table mimics the filling of the subshells with electrons. the periodic table, electron shells, and orbitals. one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce.. Subshell Blocks Periodic Table.
From saylordotorg.github.io
Electronic Structure and the Periodic Table Subshell Blocks Periodic Table one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. The shape of the periodic table mimics the filling of the subshells with electrons. the answer is rather simple, if you understand electron configurations: the periodic table, electron shells, and orbitals.. Subshell Blocks Periodic Table.
From www.thesciencehive.co.uk
Periodicity* — the science sauce Subshell Blocks Periodic Table the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. the labels s, p, d and f blocks of the periodic table refer. Subshell Blocks Periodic Table.
From utedzz.blogspot.com
Periodic Table Group 1a Periodic Table Timeline Subshell Blocks Periodic Table the labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The shape of the periodic table mimics the filling of the subshells with electrons. the answer is rather simple, if you understand electron configurations: based on electron configurations, the periodic table can be divided into. Subshell Blocks Periodic Table.
From sciencenotes.org
Periodic Table Blocks of Elements Subshell Blocks Periodic Table the periodic table is separated into blocks depending on which subshell is being filled for the atoms that belong in that section. The shape of the periodic table mimics the filling of the subshells with electrons. the periodic table, electron shells, and orbitals. one way to remember this pattern, probably the easiest, is to refer to the. Subshell Blocks Periodic Table.
From newtondesk.com
Block Classification of Periodic Table Elements Periods and Groups Subshell Blocks Periodic Table the periodic table, electron shells, and orbitals. one way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce. based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The shape. Subshell Blocks Periodic Table.